Abstract
H. pylori infection can be diagnosed using both noninvasive and invasive methods. There is no one gold standard test that is used for diagnosis. Invasive methods of diagnosis involve endoscopy with biopsy, histologic examination, culture, and rapid urease testing. There are certain clinical situations that are appropriate for noninvasive testing and invasive testing. Choosing the appropriate method of diagnosis is dependent upon multiple factors including pretest probability of Infection as well as cost-effectiveness and availability. The aim of this chapter is to discuss the current options of diagnosis, when invasive testing is indicated, and the interpretation of the results obtained.
Keywords
- H. pylori
- endoscopy
- invasive testing
- peptic ulcer disease
- urease
- culture
- histology
1. Introduction
2. Historical background of H. pylori
The discovery of
2.1 Bacteriology and pathophysiology of H. pylori
The bacteria that is responsible for infection of the stomach was originally noted as an incidental finding on many gastric biopsies. Further speciation of bacteria found that it was related to campylobacter. It was later further defined and speciated and renamed to be
There are several inherent factors that ensure survival in a hostile environment within the gastric mucosa including urease activity, motility, and the ability to adhere to gastric epithelial lining [4].
Bacterial urease hydrolyzes gastric urea to form ammonia, which facilitates invasion of the gastric mucus layer via neutralization of the acidic protective layer of the stomach [5]. The unique morphology of the spiral shaped bacterium and flagellated motility contribute to the ability to adhere to gastric mucosa.
3. Indications for testing
The established indications for testing patients for
If any of the following are identified on endoscopic evaluation: actively bleeding peptic ulcer, other endoscopic abnormality requiring gastric biopsy, or a clinical history of proton pumping inhibitor, bismuth, or antibiotic use warrant biopsy for
Medications that should be discontinued prior to
3.1 Methods of noninvasive diagnosis—Urea breath and stool antigen assay, serology
Of the myriad diagnostic tests available, all with various ranges of sensitivity and specificity, there is not one single test that can be considered a gold standard. Typically, a combination of invasive and noninvasive tests can lead to an accurate diagnosis. One of the notable disadvantages of invasive testing is the requirements of tertiary laboratory equipment and staff. Thus, several noninvasive tests have been developed.
3.1.1 Urea breath testing (UBT)
The basis of UBT involves the bacterium urease mechanism of action. Urease enzyme hydrolyzes urea within the gastric lumen to produce carbon dioxide and ammonia. The assay involves ingestion of urea with a carbon labeled isotope (typically, nonradioactive 13C or radioactive 14C). In afflicted individuals,
3.1.2 Stool antigen testing
The presence of bacterial antigen within stool samples is indicative of an ongoing age pylori infection. Thus, stool antigen testing can be used to diagnose and confirm
3.1.3 Serology
ELISA test to detect immunoglobulin G antibodies generated against
3.2 Invasive methods of diagnosis
Endoscopy is the basis for invasive methods of diagnosing
3.2.1 Histology
Historically, histologic evaluation of gastric mucosa was the first method of accurate diagnosis of
The Sydney System of classification of gastritis was originally described in 1996 and has had multiple revisions since its inception. The Sydney system classifies the severity of gastritis by the following histologic findings: the extent of lymphocyte and neutrophil invasion and the lamina propria, glandular atrophy within the corpus and antrum, intestinal metaplasia of the mucosal epithelium, and the amount of
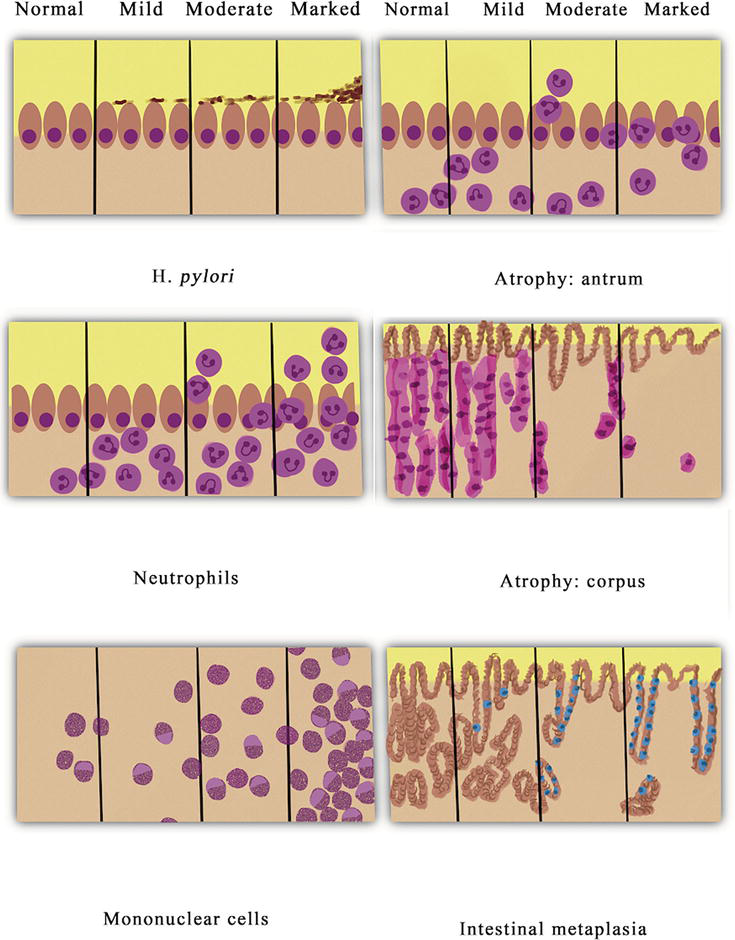
Figure 1.
Grading of gastritis by Sydney system: acute inflammation, chronic inflammation, atrophic gastritis, intestinal metaplasia, and
3.2.2 Rapid urease test (RUT)
Rapid Urease test (RUT) is another invasive method of detection of
It is notable that while both kits have sensitivity and specificity greater than 90%, there are several factors that influence these measures. The bacterial density of at least 104 organisms within the biopsy specimen is required for a positive result [22]. Additional factors that influence the specificity and sensitivity of the RUT kits include bacterial density post-treatment, patients with bleeding ulcers at the time of biopsy, patients taking H2-receptor antagonists or PPIs [23, 24]. Urease testing does have the advantage over culture and histology given the low cost, user ease, and rapidity of results.
3.2.3 Culture
3.2.4 Polymerase chain reaction (PCR)
PCR is a molecular analysis method that involves rapid production of copies of a specific segment of DNA. It uses short synthetic DNA fragments called primers to select a segment of the genome to be amplified followed by multiple rounds of DNA synthesis to amplify the segment of interest. PCR is useful in providing data involving the presence of certain virulent factors as well as antibiotic resistance [26]. An advantage of PCR is that a variety of clinical samples can be used to extract
Antibiotic resistance is one of the leading causes of failure of treatment of
4. Conclusions
There is no one gold standard test that is recommended for a diagnosis of
References
- 1.
Brown LM. Helicobacter pylori : Epidemiology and routes of transmission. Epidemiologic Reviews. 2000;22 (2):283-297. DOI: 10.1093/oxfordjournals.epirev.a018040 - 2.
Marshall BJ, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984; 1 (8390):1311-1315. DOI: 10.1016/s0140-6736(84)91816-6 - 3.
Goodwin CS, Worsley BW. Microbiology of helicobacter pylori . Gastroenterology Clinics of North America. 1993;22 (1):5-19 - 4.
Amieva MR, El-Omar EM. Host-bacterial interactions in helicobacter pylori infection. Gastroenterology. 2008;134 (1):306-323. DOI: 10.1053/j.gastro.2007.11.009 - 5.
Mobley HL. The role of helicobacter pylori urease in the pathogenesis of gastritis and peptic ulceration. Alimentary Pharmacology & Therapeutics. 1996;10 (Suppl. 1):57-64. DOI: 10.1046/j.1365-2036.1996.22164006.x - 6.
Kao CY, Sheu BS, Wu JJ. Helicobacter pylori infection: An overview of bacterial virulence factors and pathogenesis. Biomedical Journal. 2016;39 (1):14-23 - 7.
Malfertheiner P, Megraud F, O'Morain CA, Atherton J, Axon AT, Bazzoli F, et al. Management of Helicobacter pylori infection--the Maastricht IV/ Florence consensus report. Gut. 2012;61 (5):646-664. DOI: 10.1136/gutjnl-2012-302084 - 8.
DuBois S, Kearney DJ. Iron-deficiency anemia and helicobacter pylori infection: A review of the evidence. The American Journal of Gastroenterology. 2005;100 (2):453-459. DOI: 10.1111/j.1572-0241.2005.30252.x - 9.
Rostami N, Keshtkar-Jahromi M, Rahnavardi M, Keshtkar-Jahromi M, Esfahani FS. Effect of eradication of helicobacter pylori on platelet recovery in patients with chronic idiopathic thrombocytopenic purpura: A controlled trial. American Journal of Hematology. 2008;83 (5):376-381. DOI: 10.1002/ajh.21125 - 10.
Howden CW, Hunt RH. Guidelines for the management of helicobacter pylori infection. Ad hoc committee on practice parameters of the American College of Gastroenterology. The American Journal of Gastroenterology. 1998;93 (12):2330-2338. DOI: 10.1111/j.1572-0241.1998.00684.x - 11.
Vakil N, Rhew D, Soll A, Ofman JJ. The cost-effectiveness of diagnostic testing strategies for helicobacter pylori . The American Journal of Gastroenterology. 2000;95 (7):1691-1698. DOI: 10.1111/j.1572-0241.2000.02193.x - 12.
Chey WD, Wong BC. Practice parameters Committee of the American College of gastroenterology. American College of Gastroenterology guideline on the management of helicobacter pylori infection. The American Journal of Gastroenterology. 2007;102 (8):1808-1825. DOI: 10.1111/j.1572-0241.2007.01393.x. Epub 2007 Jun 29 - 13.
Patel SK, Pratap CB, Jain AK, Gulati AK, Nath G. Diagnosis of helicobacter pylori : What should be the gold standard? World Journal of Gastroenterology. 2014;20 (36):12847-12859. DOI: 10.3748/wjg.v20.i36.12847 - 14.
Lash JG, Genta RM. Adherence to the Sydney system guidelines increases the detection of helicobacter gastritis and intestinal metaplasia in 400738 sets of gastric biopsies. Alimentary Pharmacology & Therapeutics. 2013; 38 :424-431. DOI: 10.1111/apt.12383 - 15.
Lopes AI, Vale FF, Oleastro M. Helicobacter pylori infection—Recent developments in diagnosis. World Journal of Gastroenterology. 2014;20 (28):9299-9313. DOI: 10.3748/wjg.v20.i28.9299 - 16.
Rotimi O, Cairns A, Gray S, Moayyedi P, Dixon MF. Histological identification of helicobacter pylori : Comparison of staining methods. Journal of Clinical Pathology. 2000;53 :756-759 - 17.
Laine L, Lewin DN, Naritoku W, Cohen H. Prospective comparison of H&E, Giemsa, and Genta stains for the diagnosis of helicobacter pylori . Gastrointestinal Endoscopy. 1997;45 :463-467 - 18.
Genta RM, Robason GO, Graham DY. Simultaneous visualization of helicobacter pylori and gastric morphology: A new stain. Human Pathology. 1994;25 (3):221-226. DOI: 10.1016/0046-8177(94)90191-0 - 19.
Stolte M, Meining A. The updated Sydney system: Classification and grading of gastritis as the basis of diagnosis and treatment. Canadian Journal of Gastroenterology. 2001; 15 (9):591-598. DOI: 10.1155/2001/367832 - 20.
Kato M, Shimizu Y, Kobayashi T, Komatsu Y, Takeda H, Sugiyama T, et al. Rapid urease test. Nihon Rinsho. 2003; 61 (1):72-78 Japanese - 21.
Mcnicholl AG, Ducons J, et al. Helicobacter pylori study Group of the Asociación Española de Gastroenterología (AEG). Accuracy of the ultra-rapid urease test for diagnosis ofhelicobacter pylori infection. Gastroenterología y Hepatología. 2017;40 (10):651-657. English, Spanish. DOI: 10.1016/j.gastrohep.2017.07.007 - 22.
Puetz T, Vakil N, Phadnis S, Dunn B, Robinson J. The Pyloritek test and the CLO test: Accuracy and incremental cost analysis. The American Journal of Gastroenterology. 1997; 92 (2):254-257 - 23.
Mégraud F. Advantages and disadvantages of current diagnostic tests for the detection of helicobacter pylori . Scandinavian Journal of Gastroenterology. Supplement. 1996;215 :57-62. DOI: 10.3109/00365529609094536 - 24.
Lerang F, Moum B, Mowinckel P, Haug JB, Ragnhildstveit E, Berge T, et al. Accuracy of seven different tests for the diagnosis of helicobacter pylori infection and the impact of H2-receptor antagonists on test results. Scandinavian Journal of Gastroenterology. 1998;33 (4):364-369. DOI: 10.1080/00365529850170982 - 25.
Mégraud F, Lehours P. Helicobacter pylori detection and antimicrobial susceptibility testing. Clinical Microbiology Reviews. 2007;20 :280-322 - 26.
Saez J, Belda S, Santibáñez M, Rodríguez JC, Sola-Vera J, Galiana A, et al. Real-time PCR for diagnosing helicobacter pylori infection in patients with upper gastrointestinal bleeding: Comparison with other classical diagnostic methods. Journal of Clinical Microbiology. 2012;50 :3233-3323 - 27.
Cover TL. Perspectives on methodology for in vitro culture of helicobacter pylori . Methods in Molecular Biology. 2012;921 :11-15. DOI: 10.1007/978-1-62703-005-2_3 - 28.
Rimbara E, Sasatsu M, Graham DY. PCR detection of helicobacter pylori in clinical samples. Methods in Molecular Biology. 2013;943 :279-287. DOI: 10.1007/978-1-60327-353-4_19 - 29.
Gong RJ, Xu CX, Li H, Liu XM. Polymerase chain reaction-based tests for detecting helicobacter pylori clarithromycin resistance in stool samples: A meta-analysis. World Journal of Clinical Cases. 2021;9 (1):133-147. DOI: 10.12998/wjcc.v9.i1.133 - 30.
Kwon DH, Kato M, El-Zaatari FA, Osato MS, Graham DY. Frame-shift mutations in NAD(P)H flavin oxidoreductase encoding gene (frxA) from metronidazole resistant helicobacter pylori ATCC43504 and its involvement in metronidazole resistance. FEMS Microbiology Letters. 2000;188 :197-202 - 31.
Jenks PJ, Ferrero RL, Labigne A. The role of the rdxA gene in the evolution of metronidazole resistance in helicobacter pylori . The Journal of Antimicrobial Chemotherapy. 1999;43 :753-758 - 32.
Dailidiene D, Bertoli MT, Miciuleviciene J, Mukhopadhyay AK, Dailide G, Pascasio MA, et al. Emergence of tetracycline resistance in helicobacter pylori : Multiple mutational changes in 16S ribosomal DNA and other genetic loci. Antimicrobial Agents and Chemotherapy. 2002;46 :3940-3946 - 33.
Moore RA, Beckthold B, Wong S, Kureishi A, Bryan LE. Nucleotide sequence of the gyrA gene and characterization of ciprofloxacin-resistant mutants of helicobacter pylori . Antimicrobial Agents and Chemotherapy. 1995;39 :107-111